We manufacture life-saving therapies
NBI has a specialised manufacturing facility, which is the only one of its kind in Africa. The facility is current Good Manufacturing Practice (cGMP) compliant and produces a comprehensive range of plasma-derived medicinal products (PDMPs). Both NBI’s facility and its products are approved by the South African Health Products Regulatory Authority (SAHPRA). Product safety, quality and performance is our priority.
Features of NBI’s state-of-the-art manufacturing facility include:


Features of NBI’s state-of-the-art manufacturing facility include:
-
Dedicated manufacturing units:
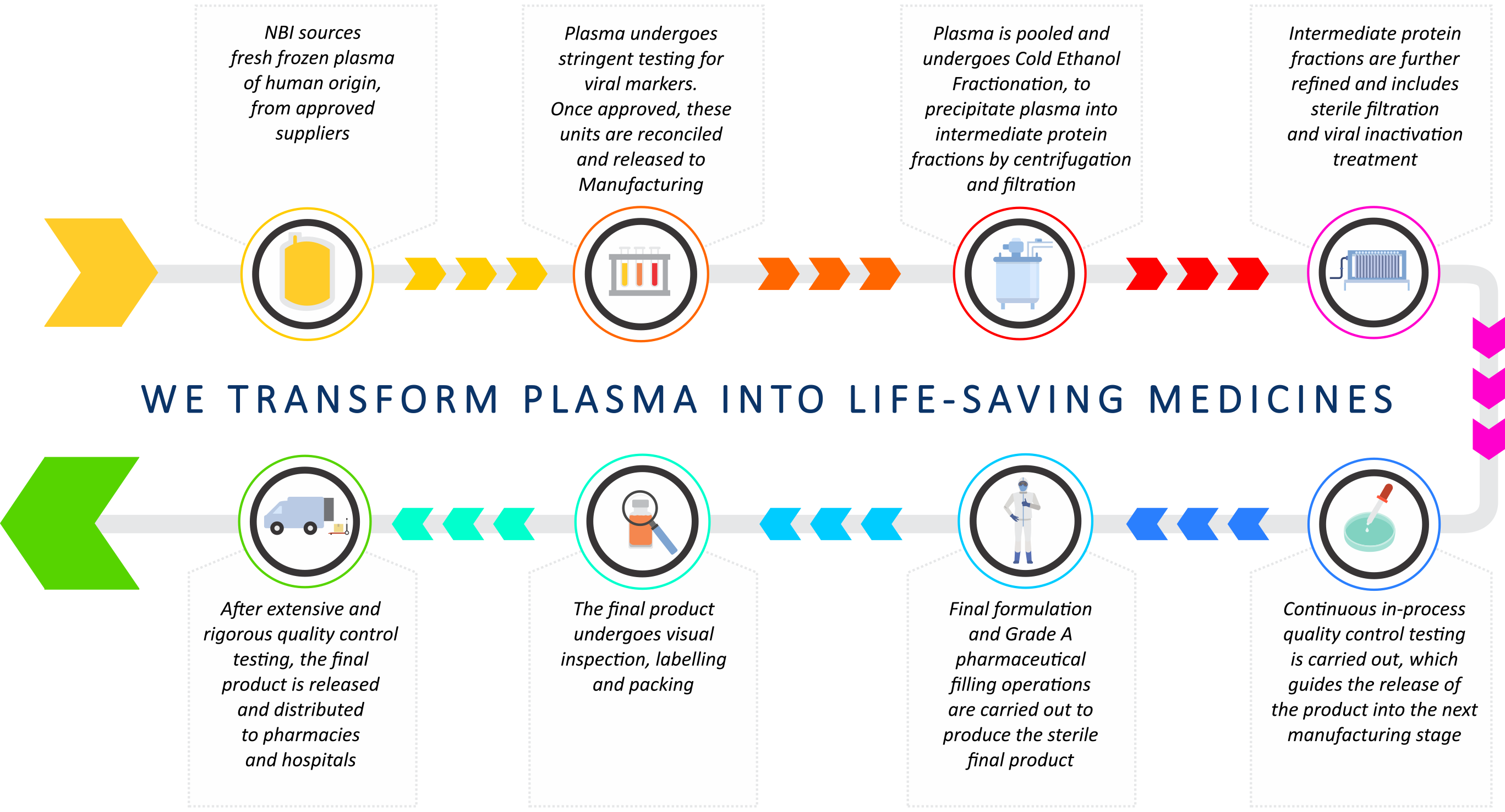
- A Fractionation unit to process human plasma into intermediate protein fractions
- A Refining unit to purify and process the intermediate protein fractions to final product specifications
- A Pharmaceutical unit for final formulation and sterile filling processes
- An inspection, labelling and packing unit
- A Biotechnology department for recombinant and monoclonal antibody production
- A Biopharmaceutical Product Development Laboratory that is equipped for product and process development
- Specially designed fractionation and manufacturing equipment
- HVAC controlled processing
- Dedicated temperature-controlled storage areas for raw materials, intermediates and final product
- SAP inventory management
- Track and trace capability (from raw materials to final product)
How we transform plasma into life-saving therapies
NBI extracts and purifies delicate therapeutic proteins from human plasma to manufacture PDMPs that meet the required standards of safety, quality and efficacy. The availability of PDMPs ultimately translates into improved access to essential treatments for patients in critical care settings, as well as for the management of patients with life-long and rare diseases. NBI manufactures a comprehensive range of PDMPs. Click here for more..
Product Safety
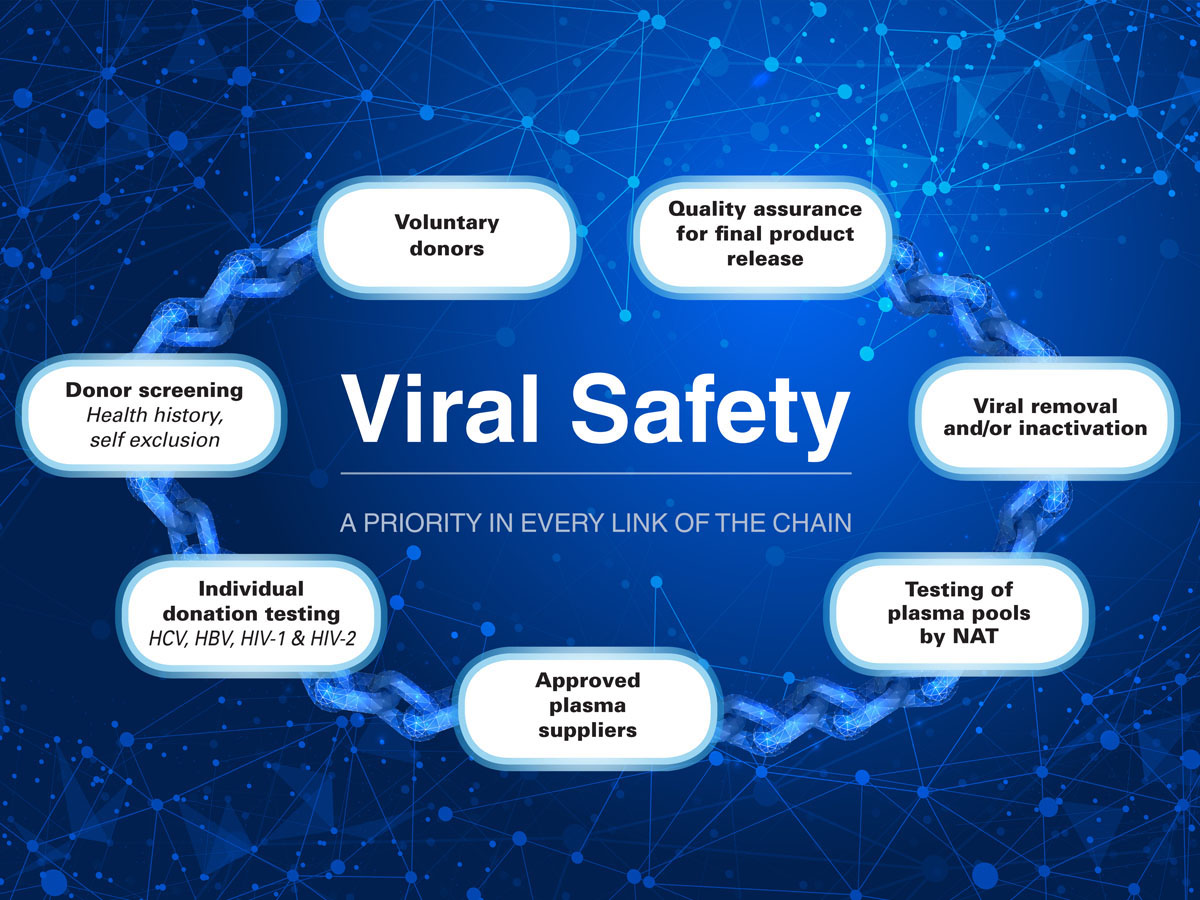
Product Safety
- Our Plasma is sourced from a broad base of healthy donors.
- Each individual donation is tested by the blood services and additional testing is performed on the pooled plasma by NBI.
- Manufacturing processes include validated, effective viral inactivation steps.
- Our product range meets international quality standards.
Quality
NBI has a firm commitment towards quality, therefore the Quality Management Systems (QMS) in place are aimed at assuring that the desired product quality, safety and efficacy is routinely met, suitable process performance is achieved, the set controls are appropriate, and continual improvement opportunities are identified and evaluated. The QMS at NBI applies to all areas of the business and has been implemented to ensure compliance to cGMP requirements as well as utilizing many of the elements of ISO systems.
NBI is certified in the following standards:
- EN ISO 13485:2016 certified (#19 0057 SJ)
- ISO 14001:2015 and ISO 45001:2018 certification
Quality
Regulatory compliance

Regulatory compliance
NBI operates in a highly regulated pharmaceutical environment complying with, inter alia, the requirements of the Medicines & Related Substances Act (Act 101 of 1965), Pharmacy Act, 1974 (Act No. 53 of 1974), SAHPRA and the South African Pharmacy Council (SAPC) Guidelines, as well as international quality standards.
- SAHPRA Licence to Manufacture Medicines (#0000000611)
- SAHPRA Licence to Manufacture Medical Devices (#00000060MD)
- Current Good Manufacturing Practice (cGMP) certification
- Department of Health Manufacturing Pharmacy Licence (KZN00011M)
- SAPC Manufacturing Pharmacy certification (Y54809)













